Packaging for Men’s Prescription Medication for Low-T
Bringing a new pharmaceutical product online and on to shelves.
Background
In 2018 Antares Pharma received FDA approval and launched the first and only weekly auto-injector for testosterone replacement therapy. We were asked to bring it online and onto shelves.
Project Objective
Use a logo provided by the client to design and develop the trade dress: physical labels, packaging and website for the new to market medication.
Brand Development Process
Discovery & Research: Conducted interviews with stakeholders to uncover the brand’s core values and unique selling points. The target market: men in middle age with low testosterone caused by hypogonadism and their physicians and healthcare providers. Everything we developed for this product was stringently reviewed by medical, legal and regulatory experts in their field and all were eventually approved by the FDA before hitting the market. Our experience understanding the barriers and guardrails to provide approvable design allowed for a successful end product for our client.
Trade Dress Design: Using the logo and style guide already developed, we designed concepts for the packaging which includes: Shelf Cartons, Device Labels, Medical Guide, IFU
Website Design & Development: For launch we developed a website that provided information for two audiences: The Patient and The Healthcare Provider.
Packaging / Trade Dress Components
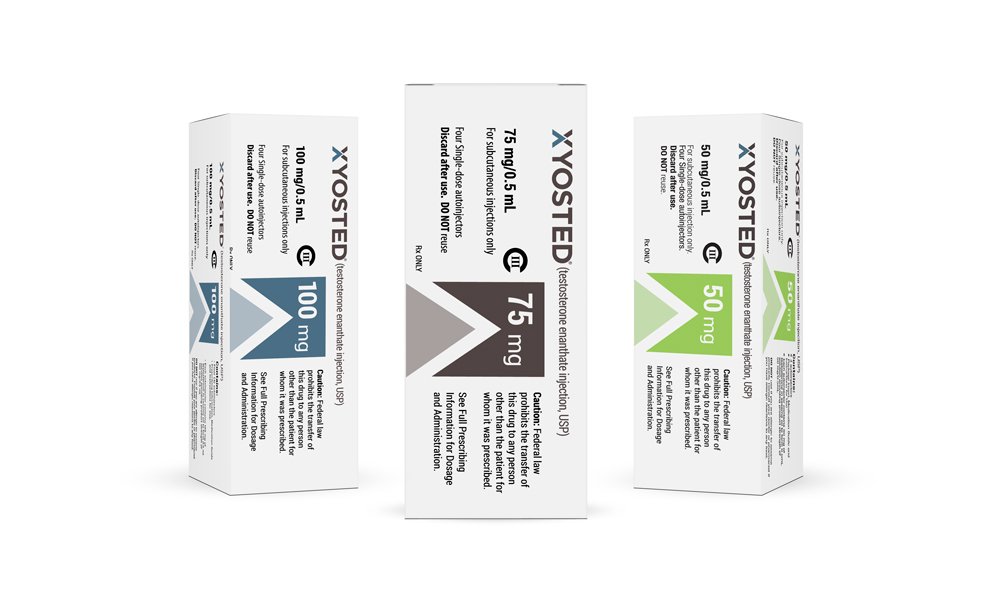
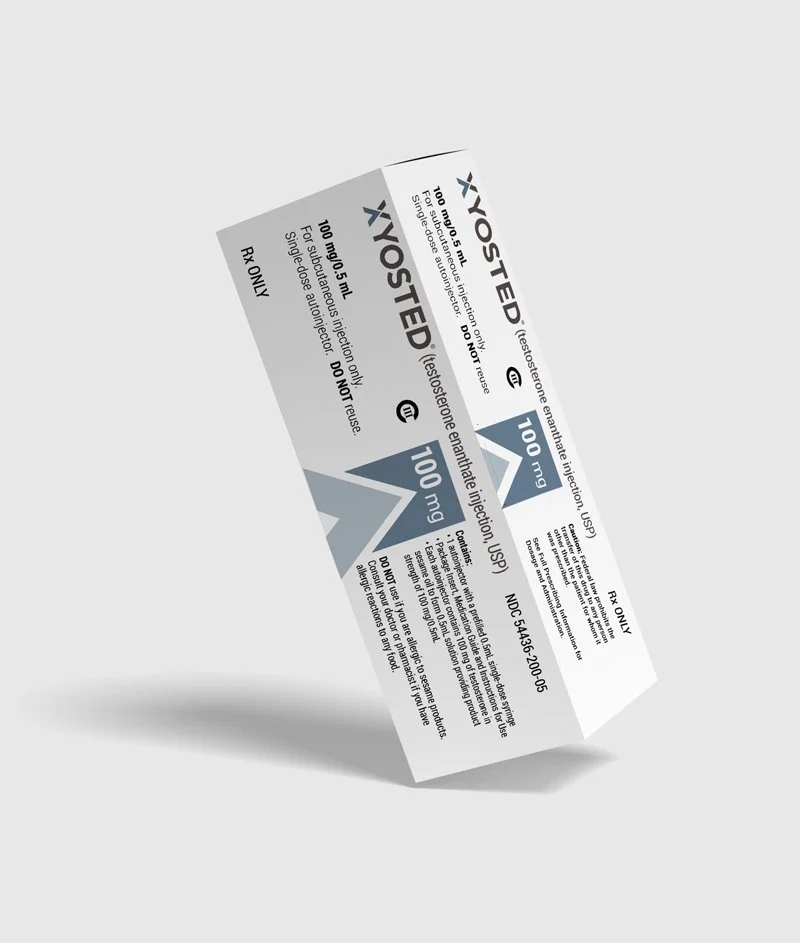
Device Labels: Designed Labels for the auto-injector device in three dose strengths, each strength using a different brand color. Additionally designed trainer labels for the product.
Multi and Single Pack Cartons: Designed matching cartons for 4 packs as well as sample single packs for each dose strength.
Medical Guide and Illustrations: Created FDA approved medical illustrations, used as figures in the product Medical Guide
IFU: Layout of the Instructions for Use, and subsequent reworks.
Results
This innovative product continues to gain market share. The men’s health industry is booming, and options for Low-T treatments using TRT continues. Our work lead to many printed and digital assets driven off of the original packaging design.
Our work continues. We assist the client on new marketing pieces for this product, using both print and digital strategies of our own creation and working with outside agencies partnered with the client.
Conclusion
Our experience in understanding the barriers and guardrails allowed us to provide FDA approvable design options and a successful end product for our client.
The packaging we designed is still being used today on pharmacy shelves and doctors sample closets. We continue to work with the client on edits, and changes as needed by the changing FDA regulations. Eventually the website went through a number of iterations of our marking and recently our client partnered with another agency to design a new website which we coded, tested and launched in partnership with them. The new website included a brand refresh.